


Principal Investigator
Dr. Deborah Schechtman
Deborah Schechtman (IQ-USP)
CV Lattes
ORCID
Web of Science
 |
 |
|
Deborah Schechtman, obtained her BA in Biological Sciences (Biomedidine) from the Universidade Federal do Estado do Rio de Janeiro (Uni-Rio), masters in Biological Siences Biológicas (Biophysics) from the Universidade Federal do Rio de Janeiro, and PhD in the department of Chemical Immunology at The Weizmann Institute of Science. Accomplished her Post doctoral studies at the University of Stanford. Was a Young Investigator (FAPESP) at the Heart Institute (InCor/ HCFMUSP) at the Laboratory of Genetics and Molecular Cardiology. Presently is an associate Professor at the Chemistry Instituto Biochemistry Department of the Universidade de São Paulo, working on signal transduction pathways leading to pain. The Laboratory of Biochemistry of Cell Signaling is localized at LAPIC, Chemistry Institute of the University of São Paulo and has as a main goal understand the signal transduction pathways involved in pain for the development of new non-opioid analgesics. Analyzing mutations in TrkA the tyrosine kinase coupled e high affinity NGF receptor, in patients with congenital insensitivity to pain with anhidrosis (CIPA), and thus these patients don't feel pain, we identified the PLCg pathway activated by TrkA as a key pathway for pain signaling. We developed a peptide that inhibits this signaling pathway. This peptide has an analgesic effect in animal models of inflammatory pain. Using multidisciplinary approaches that involve structural biology, physiology, embryology, biochemistry, chemistry and cell biology and several collaborators in Brazil and world wide we are looking at the effects of this peptide in other animal pain models, understanding how mutations in TrkA can affect the development of the peripheral nociceptive nervous system and identifying other signaling pathways that may be affected by the peptide, and characterizing the effect of this pathway in pain. We are also developing strategies to find small molecules that can act like the peptide and can serve as leads to develop new analgesics. |
|
People
Postdocs |
||
 |
The effect of mutations of the gene NTRK1 found in patients with Congenital Insensitivity to pain in the nociceptive nervous system. Develop a system to study the effects of mutations on the gene NTRK1 que that leads to congenital insensitivity to pain with anhidrosis (CIPA) in the development of nocicepors in chicken embryos. Dr. Felipe Monteleone Vieceli Este endereço de email está sendo protegido de spambots. Você precisa do JavaScript ativado para vê-lo. CV Lattes |
|
PhD Students |
||
 |
Searching for small molecules that interfere with the TrkA/ PLCγ interation Our group establishmed that the protein/ protein interaction between TrkA and PLCγ is a target for new analgesics, making it important to study the specificity of PLCγ 's SH2 domains in the recognition of TrkA. Besides studying the specificity of PLCγ 's SH2 domains for diverse peptides derived from growth factor receptor coupled tyrosine kinases we search for new strategies to find modulators for this interaction. In a fragment library screening assay we found algumas candidate molecules to be validated both structurally and functionally. We can chemically improve these molecules to test them in biochemical and in vivo assays. Msc. Allan Pradelli Roldão Este endereço de email está sendo protegido de spambots. Você precisa do JavaScript ativado para vê-lo. CV Lattes |
 |
 |
|
 |
Masters Students |
||
 |
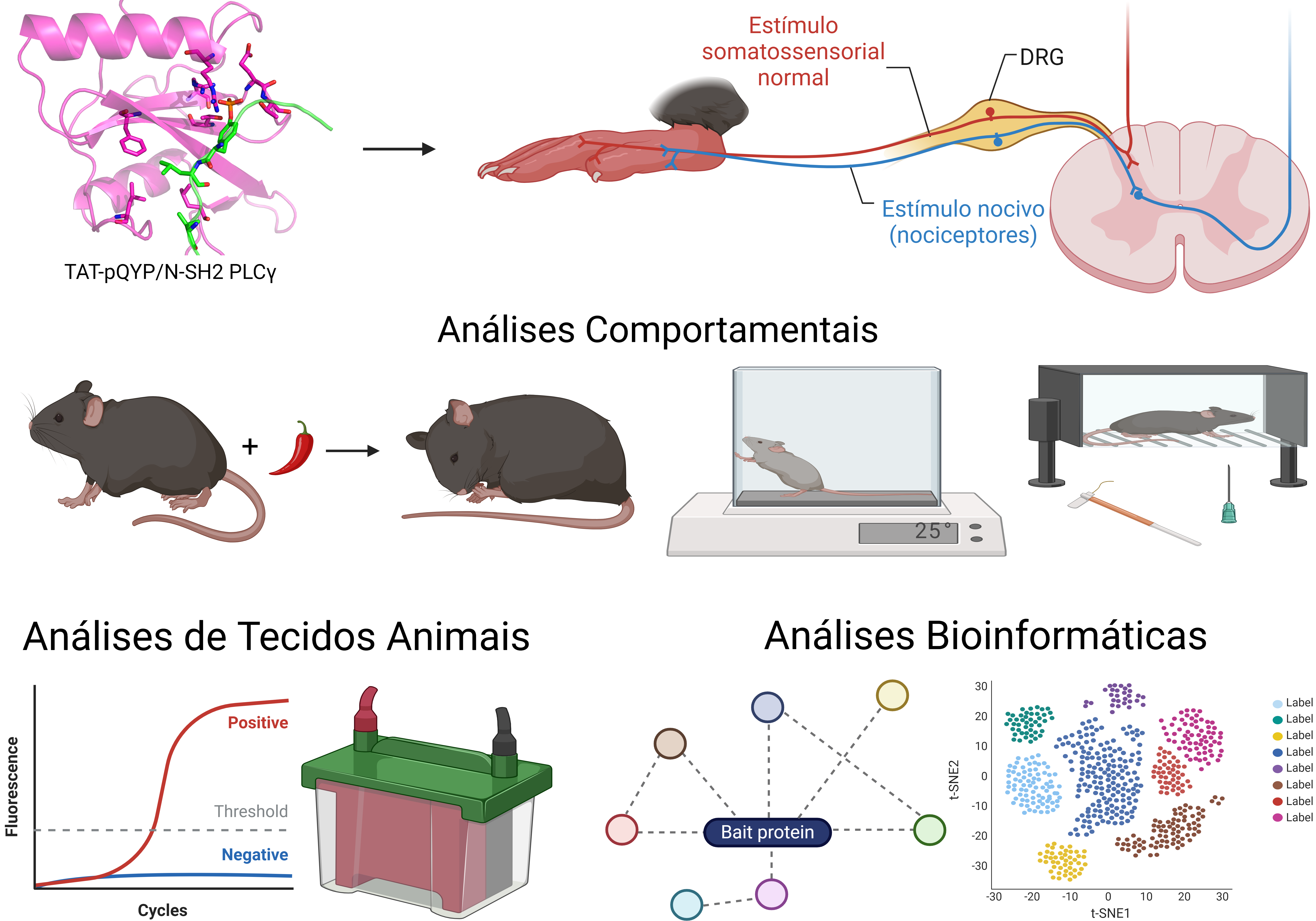
Effect of the interaction between TrkA and PLCg in peripheral hypersensitivity signaling mediated by TRPV1 Understanding how nococeptive signaling occurrs is fundamental for the development of new analgesics, necessary considering the low efficiency of the available opioids. The objective of my project is to evaluate d PLCγ dependent and independent mechanisms for peripheral sensitization mediated by TRPV1, besides it's role in the associated inflammation. Using a variaty of inflammatory hipersensitivity models evaluating behaviour as a main strategy to investigate the role of signaling pathways in analogous pain phenotypes. Furthermore, human and mice transcriptome data available and tools to modulate specific proteín/ proteín interactions are used to elucidate the role of e PLCγ in nociceptive signaling pathways Alexandre Martins do Nascimento Este endereço de email está sendo protegido de spambots. Você precisa do JavaScript ativado para vê-lo. CV Lattes |
 |
Undergraduate Students |
||||||||||||||||||||||||||||||||||||||||||||||||
 |
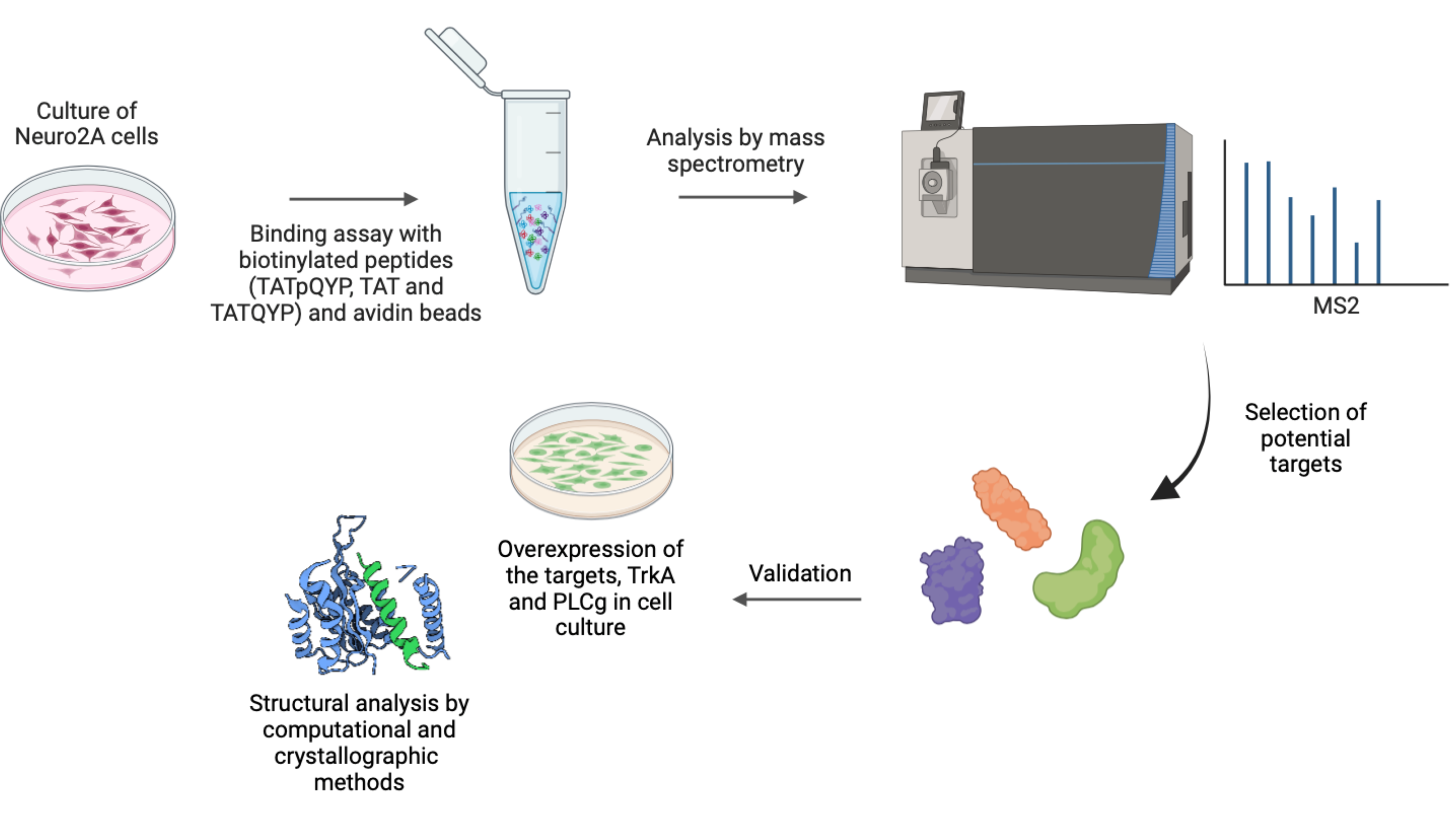
Identification of proteins that bind to TAT-pQYP My project aims to find proteins that bind to the peptide TAT-pQYP, besides PLCg that may contribute to the analgesic effect of the peptide. To this end, we used affinity columns with the biotinilated peptide followed by mass spectrometry, to identify binding proteins. Binding proteins are then validated as potential targets and characterized as well as the signaling pathways involved in the analgesic effect of TAT-pQYP, and potential side effects caused by the peptide. |
 |
||||||||||||||||||||||||||||||||||||||||||||||
 |
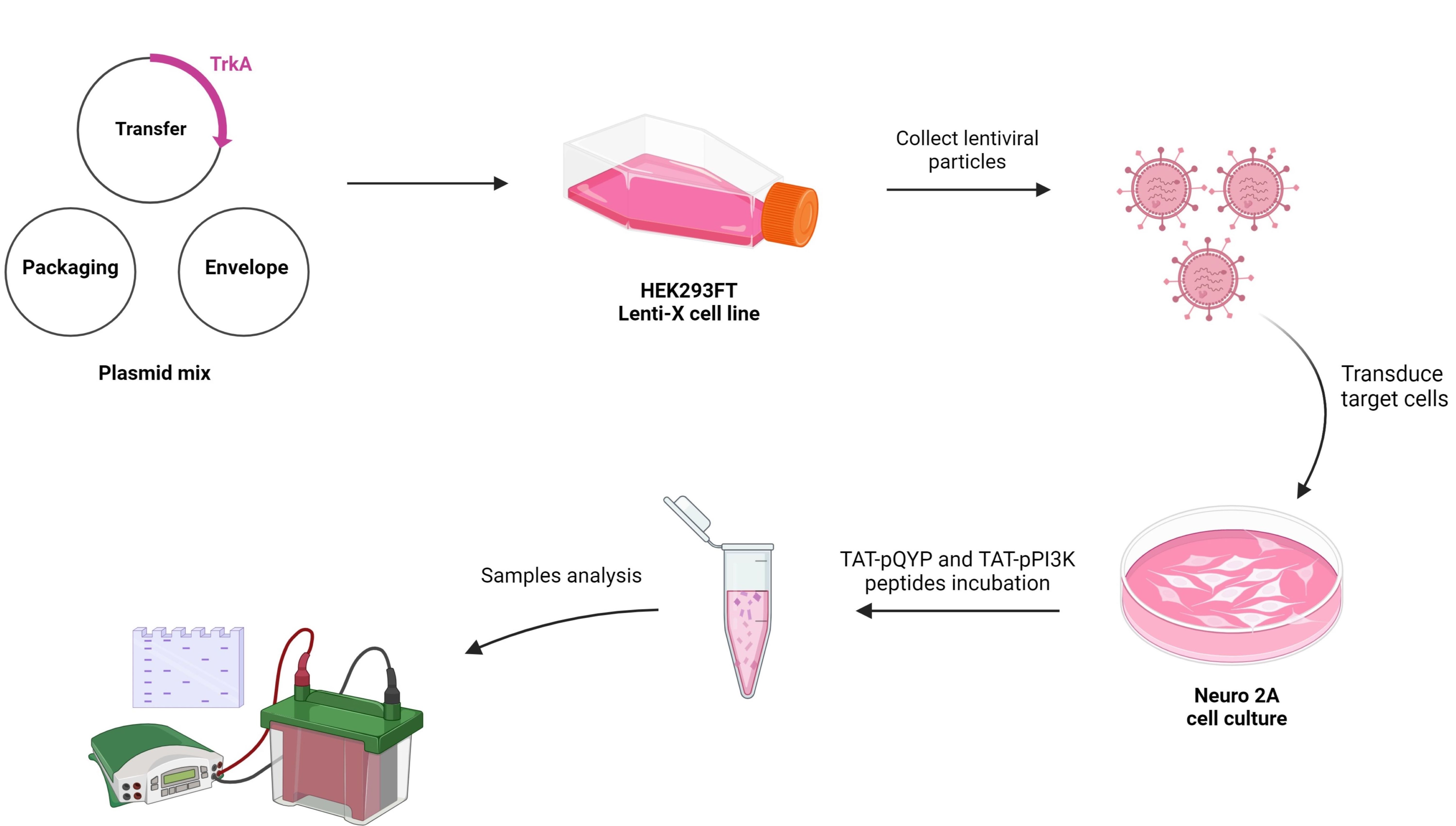
My project we will perform an in depth analysis of the PI3K and PLCg sigaling pathways that are implicated in pain signaling. Therefore, we wish to test cell permeable phosphopeptides capable of biding to their respective domains in these pathways inducing modulatios that will permit to better describe their role in cell processess. such as neurite outgrowth, and interaction with other proteins related to TrkA. To this end we will develop a cell line usig Neuro2A cells, that overexpresses TrkA. This cell line will also help us test the function of small molecule modulators of TrkA-PLCg interactions as described above. Giovanna Souza Stival |
 |
||||||||||||||||||||||||||||||||||||||||||||||
Technicians |
||||||||||||||||||||||||||||||||||||||||||||||||
 |
As a Lab Manager, I assist undergraduate and graduate students in conducting their experiments. My work involves practical guidance, problem-solving, and teaching essential experimental techniques. I also plan and organize some of the experiments conducted in the laboratory, ensuring that all steps are followed correctly and that the results are accurate and reproducible. Additionally, I am involved in an innovative project focused on the implementation of new drugs for pain treatment. This project not only expands our scientific knowledge but also has the potential to positively impact patients' quality of life. Overall, my role is to ensure that the laboratory operates smoothly and efficiently, facilitating high-quality research and contributing to scientific advancement in our field. Dr. Ana Maria Rodrigues Lab Manager Ana Maria Rodrigues CV Lattes |
 |
||||||||||||||||||||||||||||||||||||||||||||||
Some publications from our group |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||









